Discover the Science Behind PathFinder

Choline, Sulfur and Carbon Donation
PathFinder provides nutrients involved in Choline/Methionine/One-Carbon metabolic pathways, three powerful metabolic pathways that are important parts of liver metabolism.. These three pathways work together in a tightly-controlled manner. Homeostasis in these metabolic pathways is vital for liver health (see Diagram 1, right, click on diagram to see full-size image).*
The metabolic map on the right shows both biosynthetic pathways for phosphatidylcholine production, methionine metabolism, including transsulfuration for glutathione biosynthesis, and how they are all regulated and controlled by one-carbon metabolism including the folic acid cycle and betaine from choline metabolism.
One-Carbon (…to Rule Them All)
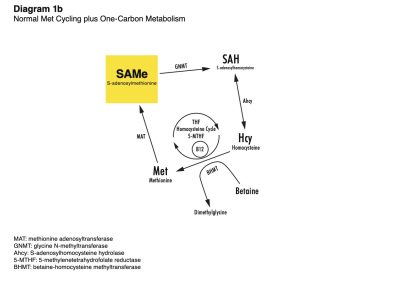
Lord-of-the-Rings metaphors aside, one-carbon metabolism (also known as methyl donation) exerts extraordinary control over the methionine and choline pathways. One-carbon metabolism refers to the transfer of single carbon molecules (methyl groups) through the body in a process called transmethylation.*
One-carbon metabolism includes the folic acid cycle and betaine, either of which which may donate a carbon molecule (transmethylation) to homocysteine (HCY) in order to convert it back to methionine (MET).*
The most prolific methyl donor is S-adenosylmethionine (SAM). SAM is involved in over 100 transmethylation reactions, including phosphatidylcholine (PC) production, protein modification, and DNA methylation (a process that controls epigenetic gene expression) and more (see Diagram 1b, right, click on diagram to see full-size image).*
Glutathione (Transsulfuration)
The Second Fate of Homocysteine*
Depletion of dietary methyl donors causes depletion of glutathione due to depletion of SAM.*
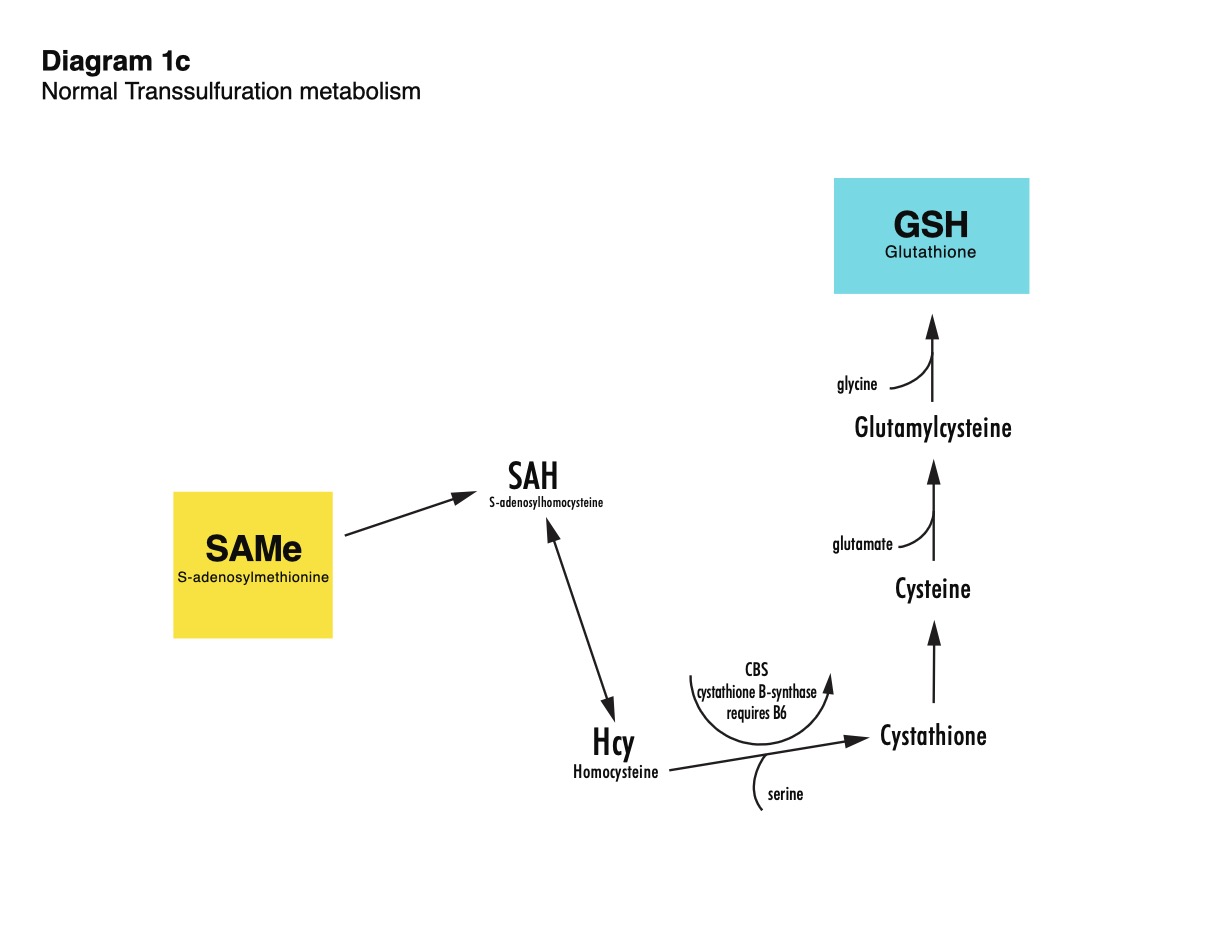
Homocysteine sits at the crossroads of Transsulfuration and Transmethylation (see diagram, right). Transsulfuration is the process of Glutathione (GSH) biosynthesis. Glutathione is a sulfur containing tripeptide composed of L-Cysteine, L-Glutamate and L-Glycine.*
Glutathione is the body’s master antioxidant and it exerts control over the body’s oxidation/reduction (REDOX) balance. Glutathione also performs detoxification duties, scavenging heavy metals from the body. GSH is essential for cellular redox homeostasis and GSH synthesis is tightly regulated in the cytosol.*
The production of glutathione involves incorporating three amino acids—L-cysteine, L-glycine, and L-glutamate—into a tripeptide within the cytosol of cells. L-cysteine availability is a key factor in this process, as it is the rate-limiting step for glutathione production.*
PathFinder provides N-acetylcysteine, a bioavailable form of L-cysteine, as well as L-glycine and L-glutamate to support normal glutathione production (please see diagram 1c, right, click on image for full size).*
Phosphatidylcholine(phospholipid) production
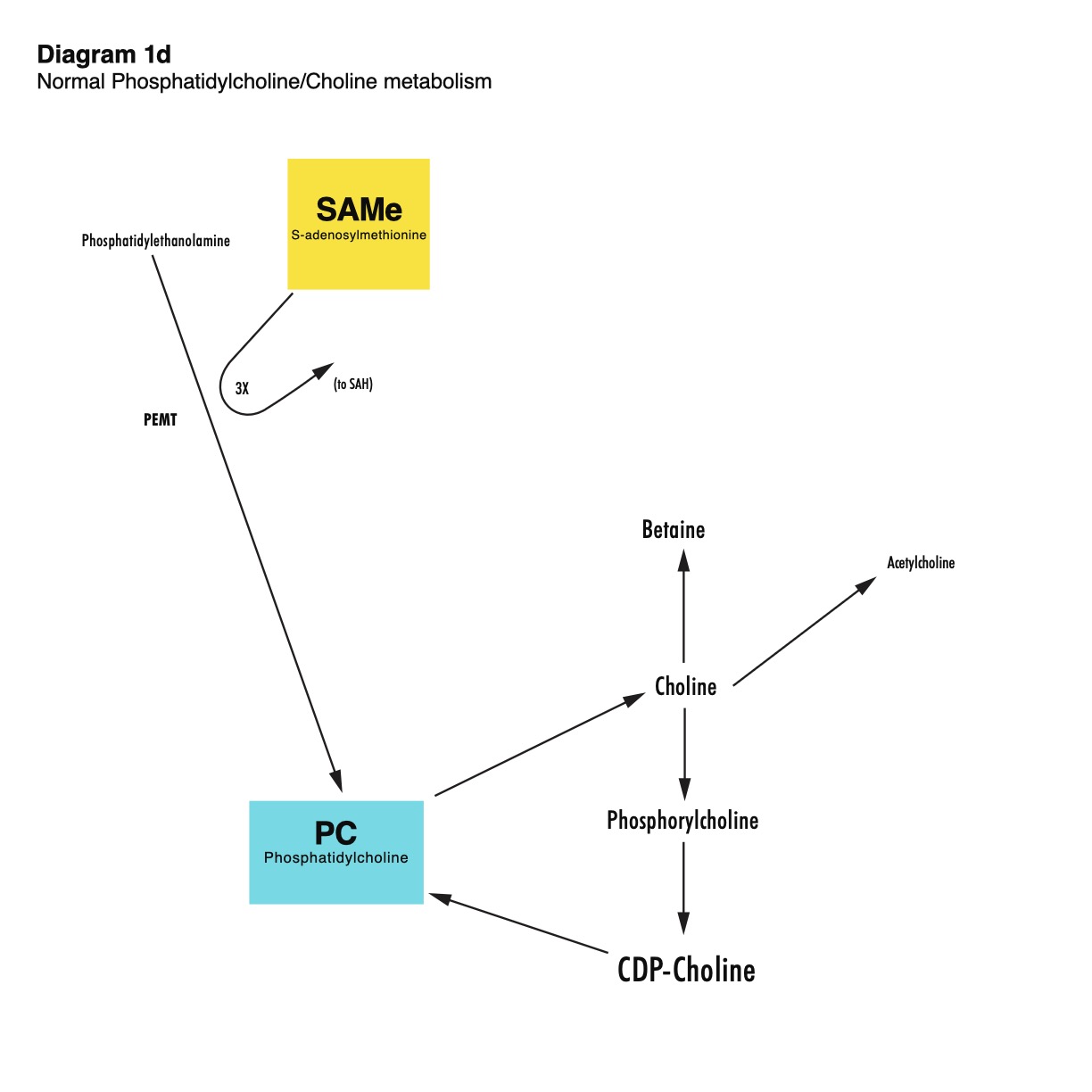
Phosphatidylcholine (PC) is a major biosynthetic product of choline metabolism and makes up about 80% of our cell membranes. It plays a key role in lipid transport throughout the body, cell signalling and cell membrane integrity.
While PathFinder does not contain PC, we offer PPC as a complementary product in a special combination with PathFinder. PPC provides additional support for choline and phosphatidylcholine metabolism, complementing PathFinder’s support for methionine and one-carbon metabolism. Together, these linked pathways help support normal liver metabolism and promote overall liver health (please see diagram 1d, right, click on image for full size).*
Mitochondrial metabolism
PathFinder provides a comprehensive blend of nutrients, including Acetyl L-Carnitine, CoQ10, alpha-lipoic acid, B vitamins, vitamin E, selenium, molybdenum, and zinc, to support healthy mitochondrial function. Mitochondria are the energy powerhouses of the body, playing a key role in normal metabolic processes. PathFinder is formulated with nutrients that help maintain mitochondrial health and function, promoting overall energy production and metabolic support.*
Small-molecule antioxidants work in cooperation to support the body’s natural antioxidant systems. In Liver Fibrosis they are depleted across the board.*
Small-molecule non-enzymatic antioxidants are critical players in protecting biological systems against oxidative damage. These molecules neutralize reactive oxygen species (ROS) and free radicals, preserving cellular integrity.
Antioxidants may only quench one free radical in their reduced form. After quenching a free radical many of these antioxidants must be converted back to a reduced state in order to regenerate antioxidant activity again. PathFinder supports this system by supplying a broad range of cooperative vitamins, minerals, botanical ingredients, and sulfur-containing antioxidants including alpha-lipoic acid and N-acetylcysteine to promote glutathione production.*